Raman Spectroscopy Proves the Presence of Single Layer Graphene Oxide
Raman spectroscopy has been used to prove William Blythe's graphene oxide readily disperses into monolayers.
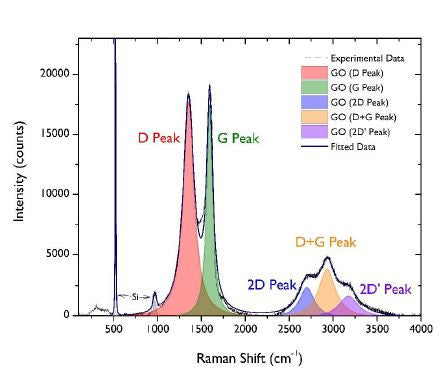
Raman spectroscopy is a well-known and very common technique for the analysis of graphene related materials. By assessing the position, intensity and ratios of the D and G peaks, it is possible to learn a lot about the material in question. Useful information from raman spectroscopy include the level of oxidation and whether the graphene oxide is present as single, double or few layer material. The ratio of the D and G peak intensities (ID/IG) can be used to estimate the distance between defects, where the term defects refers to disruption in the bonding structure observed for pristine graphene. When analysing graphene oxide, the number of defects is expected to be high as each oxygen functional group on the surface increases the amount of sp3 hybridisation and therefore reduces sp2 hybridisation.
Recent raman spectroscopy carried out on William Blythe’s graphene oxide proved that the material was fully oxidised with an ID/IG ratio of about 1, which is fairly typical for graphene oxide. An estimation of the number of layers present can be given by comparing the area of the D and G peaks to the area of the silicon substrate peak (ca 950 cm-1). Coupling with an SEM image and applying false colour, it has been possible to illustrate the presence of large quantities of single layer graphene oxide. While some 2 layer and 3+ layer material is present, based on the flake shapes and the nature of spin coating, it is thought that these areas are more likely to be flake overlaps rather than multilayer graphene oxide.
The raman analysis carried out on William Blythe’s graphene oxide shows that the graphene oxide manufactured readily exfoliates into monolayers in aqueous dispersions. This has previously been indicated by the ease of dilution. If you have any questions about this analysis or how you can incorporate graphene oxide into your work, please get in touch.